Introduction
Steel often requires heat treatment to obtain improved properties e.g increase hardness or strength, or to neutralise
negative effects resulting from previous manufacturing processes e.g.remove internal stresses generated by fabrication processes.
The various heat treatment processes include.
- Normalising
- Anealing
- Hardening
- Tempering
- Refining
- Sub-critical Anealing
|
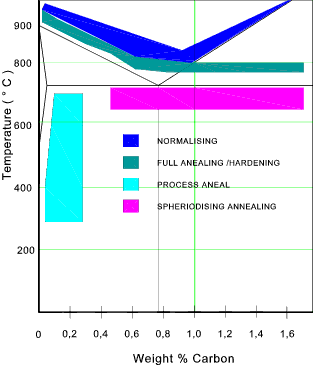
Normalising
Normalising involves heating the steel to about 40oC above its upper
critical limit. The steel is then held at this temperature for a period of
time and is then cooled in air.. It is desireable that the temperature
of the steel shall be maintained for a time period more than 2 minutes per mm of
section thickness and shall not exceed the upper critical temperature
by more than 50oC.
The structure produced by this process is pearlite (eutectoid) or pearlite in a ferrite matrix (hypoeutectoid)
or pearlite in a cementite matrix (hypereutectoid). Because the steel is cooled in air
the process results in a fine pearlite formation with improved mechanical properties compared
to the full annealing process below
Normalising is used to
- To refine the grain structure and to create a more homogeneous austenite when a
steel is to be reheated for quench hardening or full annealing
- To encourage reduced grain segregation in castings and forgings and provide a more uniform structure
- To provide moderate hardening
|
Full Anealing
Anealing is reheating steel followed by slow cooling. It is completed
a) to remove internal stress or to soften or
b) to refine the crystalline structure (This involves
heating to above the upper critical temperature ).
The steel is heated about 25oC above the upper critical temperature,
held for a set time and then cooled slowly in the furnace. This process is used
to remove internal stresses built up as a result of cold working and fabrication processes.
Following annealing the dislocations are rearranged in to a lower energy configuration, new strain free grains
are formed and grain growth is encouraged.
Hardening
Hardening involves heating a steel to its normalising temperature and cooling (Quenching )
rapidly in a suitable fluid e.g oil, water or air.
Steel is basically an alloy iron and carbon some steels alloys have have various
other elements in solution. When steel is heated above the upper critical
temperature (about 760oC), the iron crystal structure will change to
face centered cubic (FCC), and the carbon atoms will migrate into the central
position formerly occupied by an iron atom. This form of red-hot steel
is called austentite (γ iron). If this steel form cools slowly,
the iron atoms move back into the cube forcing the carbon atoms back out, resulting
in soft steel called pearlite. If the sample was formerly hard, this
softening process is called annealing.
If the steel is cooled quickly (quench) by immersing it in oil or water, the carbon
atoms are trapped, and the result is a very hard, brittle steel. This
steel crystal structure is now a body centered tetragonal(BCT) form called martensite.
Stress Relieving/Recovery (Process Anealing )
This process involves heating the metal to a temperature in the range 550oC to 650oC and held
at this temperature before being cooled at a controlled rate. This also reduces stresses
resulting from cold working and fabrication by allowing dislocations to rearrange to a lower energy
configuration.
This process is used to allow further forming operations and to prevent distortion of
the steel components as a result of subsequent machining operations
Spheroidising
The process applies more to the hypereutectoid steels (above 0,8% C). The process involves
heating the metal to between 600oC and 650oC and holding it at at the
selected temperature for a period of time the cementite changes from a lamella formation
to a formation based on an alpha ferrite matrix with particles of spheroidal cementite (Fe3C)
are embedded. This resulting steel has improved ductility and toughness compared
to the original steel with reduced hardness and strength.
Tempering
Tempering is the process of reheating the steel leading to precipitation and
spheroidisation of the carbides. The tempering temperature and time are
generally controlled to effect the final properties required of the steel.
The benefits resulting are the increase in the metal toughness and elongation.
The negative effects are the reduction of the martensite (BCT) structure and the
progression towards a spheroidal carbide + ferrite matrix structure.
Hardenability
The hardenability of a steel is broadly defined as the property which determines
the depth and distribution of hardness induced by quenching. Hardenability is a
characteristic determined by the following factors
- Chemical composition
- Austenite grain size
- Structure of alloy before quenching
|
The hardenability is the depth and evenness of hardness of a steel upon quenching
from austenite.
Thickness Considerations
The properties of heat treated steel are significantly affected by the thickness of the
section. Hardening consist of heating the steel through and just above its critical range
to obtain the condition of solid solution and quenching with sufficient rapidity to retain
this condition. If a steel has a large thickness it is practically impossible to obtain an
even temperature throughout and the middle of the section is always at a lower temperature
compared to the outside surfaces. On quenching the heat is absorbed rapidly from the outside
and it is impossible even with the most drastic quench processes to remove heat from the core
region sufficient to obtain the desire structure. For thin sections it may be possible
to obtain the desire structure throughout the section with a comparative mild quenching process.
Quenching Medium
There are a number of fluids used for quenching steels listed below in order of quenching
severity
- Brine
- Water
- Oil
- Special liquids
- Air
|
Note: Agitation of medium increases its quenching severity
Soft distilled water is the preferred medium when using water for quenching carbon steels. The
water should have no impurites such as oil, grease or acids as they could result in uneven
hardening if they stick to the surface of the steel being hardened an provide local thermal insulation.
Hard water is unsatisfactory because it may release scale as the temperature is raised. Soap
is sometimes added to adjust quenching rates. Cold brine or water is used to provide
the most severe quench with the consequent maximum hardness. Extreme care is require in
the selection of sections shapes hardened as the process result in severe thermal shock with
consequent cracking and distortion.
Oil bath quenching is used where extreme hardness is not required and where freedom from
quenching shock is needed. Oils used are mainly mineral oils with the viscosity
selected to suit the type of steel to be quenched. Oil cooling systems are required
when significant quenching capacity is required to prevent the oil from breaking down and to
maintain the quenching conditions.
Air cooling is used for mild hardening process when a tough hard pearlitic structure is required.
Vacuum Treaments
Many of the heat treatment processes can be completed in vacuum furnaces at very low
pressures (high vacuums). The advantages of using vacuum furnaces
are listed below.
- No surface oxidation or discolouration
- Minimal distortion
- No post cleaning operations
- Near finished, machined shape prior to treatment
- Normally improved control of processes
|
Flame Hardening
This process involves direct an oxy acetylene flame on the surface of the steel being hardened
and heating the surface above the upper critical temperature before quenching the steel in a spray
of water. This is also known as the shorter process.
This is a surface hardening process resulting in a hard surface layer of about 2mm to 6mm deep. The main
difference between this process and other surface hardening processes is that the composition of the
steel being hardened is not changed. The steel must itself have sufficient hardenability . This limits
this process to steels having carbon contents of above 0,35%. Steels with carbon contents of 0,4%-0,7% are most
suitable for this process. Steels with higher content and high alloy steels may not
be suitable as they a liable to cracking. This process produces similar result
to the conventional hardening process but with less hardness penetration.
Induction Hardening:
Induction hardening provides a similar surface treatment regime to flame hardening .
The steel component is located inside a water cooled copper coil which has (AC)
alternating current through it. This causes the outer surface of the component to heat up.
Depending on the AC frequency and current, the rate of heating as well as the
depth of heating can be controlled. This process is well suited for surface heat treatment.
Case Hardening
The primary purpose of case hardening is to produce a surface which is resistant to wear while
maintaining the overall toughness and strength of the steel core. This type of process
is normally used on a steel with a low carbon content and introduces carbon by diffusion (carburising)
into the local surfaces requiring treatment.. Subsequent heat treatment develops the desired combination
of high surface hardness and internal toughness. Another process called Nitriding consists of
the diffusion of nitrogen.
Notes on three primary carburising processes (Pack Carburising, Gas Carburising and Liquid Carburising are
provided below.
Pack Carburising
This process is the simplest and earliest carburising process based on placing the components
to be treated in metal containers with the caburising mixture, based on powdered
charcoal and 10% barium carbonate, packed around the components. The containers are then heated
to a constant temperature (850oC to 850oC )for a time period to ensure an even temperature throughout and
sufficient to enable the carbon to diffuse into the surface of the components to sufficient depth.
Because this process is difficult to control case depths of less than 0,6mm are not viable and the
normal case depths produced are 0,25mm to 6mm.
Gas Carburising
Gas caburising allos is accurate control of the process temperature and caburising
atmosphere. The components are brought to a uniform temperature in a neutral atmosphere.
The caburising atmosphere is introduced only for the required time to ensure the correct depth
of case. The carbon potential of the gas can be lowered to permit diffusion avoiding
excess carbon in the surface layer.
Gas carburising uses a gaseous atmosphere in a sealed furnace usually
containing propane (C3H8) or butane (C4H10).
Sometimes the generted carbon dioxide, water vapour, and oxygen are controlled at low levels
by purifying using activated carbon filters at high tempertures.
An alternative carburising atmosphere is sometime generated by using a drip feed system by feed an organic
fluid based on methyl , ethyl or isopropyl achohol + benzene or equivalent is fed into the
carburising chamber at a controlled rate. In this process there are generally internal fans
working to ensure and even gas in the chamber.
After carburizing, the work is either slow cooled for later quench hardening,
or quenched directly into various liquid quenches. Quench selection is
made to achieve the optimum properties with acceptable levels of dimensional change.
Hot oil quenching is preferred for minimal distortion, but may be limited in application
by the strength requirements for the product.
Liquid Carburising
This process is mostly used for producing shallow case depths in thin sections.
The components are heated quickly in a bath containing a suitable sodium cyanide salts
and sodium carbonate. The proportion of NaCN being maintained 20% to 30% by controlled
feed strong NaCN.
The normal case depths for this process are about 0,25mm with bath strengths of 20% to 30% NaCN.
High bath strengths 40% to 50% NaCN are required for case depths of 0,5mm. The case resulting
from this process includes carbon and nitrogen. The nitrogen does provide a hard surface
but can also encourage retained undesireable austenite in the surface layer. The bath is sometimes
convered with a graphite material to reduce the nitrogen content.
This process normall works with bath temperatures of 800oC to 950oC for immersion
times from 2 to 7 hours depending on the depth required.
For thicker case depths (up to 1,6mm) activated salt baths are used these are based on cyanide and
alkaline earth chlorides which act as the activators.
Components are normally jigged and pre-heated to about 350oC before being
introduced into the bath.
Heat treatment following carburisation
The time of heat treatment post carburisation relates to the condition of the steel. If the steel
is prepared as a fine grain steel it is possible to complete a single quench operation following case
hardening. If the steel does not have a fine grain structure a normal process
is to quench from about 870oC the quench again from about 790oC . This ensures
reasonable mechanical properties in case and core.
Nitriding
Certain steel alloys can absorb nitrogen with a resulting extremely hard surface layer.
The process consists of maintaining the steel component at a carefullly controlled temperature
of 490oC to 530oC under the action of nascent of active nitrogen produced on the
surface of the component by the decomposition of gaseous ammonia. The resulting surface
is extremely hard and extremely thin but very brittle. An nitrides based on steel alloys
are less brittle and more stable than straight iron nitrides and therefor this process is only
used for certain alloy steels..
The process time is relatively long compared to the carburising process at about 90 hours.
The temperature of the furnace has to be maintained within ±5oC and therefore electrical heating is
generally used. The components are generally stacked in gas-tight boxes supported
on nickel mesh trays. The boxes include a inlet and outlet pipes for the ammonia gas
circulation flow.
Quenching is not normally required following nitriding and therefore is normal to machine
the components to size before nitriding. Nitriding does involve small dimension
increases of up to (0,05mm) on diameters and smaller amounts on individual flat surfaces and
lengths.
Nitrided surfaces retain hardness even if cycled for short periods at temperatures of
up to 500oC. Carburised hardened surfaces lose their hardness under similar
circumstances.
Steel subject to nitriding is generally hardened and tempered and finished machined. The components
are often stress relieved prior to final machining. The nitriding process is also often followed
by surface grinding to remove the most brittle outer layer.
|
