Introduction
This page includes notes on iron and what happens when iron /carbon mixtures are cooled
from liquid to solid. The notes are based on the Iron Phase Diagram
(equilibrium diagram ). A "Phase" is a form of material having characteristic structure and properties. It is a form of
the material which has identifiable composition, structure and boundaries separating it from other
phases in the material volume. The diagram below shows shows the phases present when
when Fe-C alloys (C up to 7%) are cooled from liquid to solid.
The left side of the diagram represents pure iron and the right hand of the diagram
represents an alloy with 6,67% C. which result on cooling in the formation of Cementite.
This is a intermetallic compound (iron carbide-Fe3 C) which although not 100% stable is
but is to all practical purposes a stable phase. The phase diagram shown is therefore
a meta-stable phase.
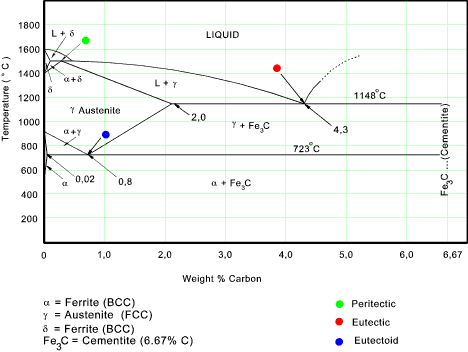
Note: Different reference sources indicate the Eutectoid point at 0,8% C and 0,77% C.
Iron Forms
Iron can exist in three forms:
α... BCC crystal with crystal dimension a = 2,86 Angstrom exists at temperatures up to 910oC
γ... FCC crystal with crystal dimension a = 3,65 Angstrom exists at temperature range 910oC to 1403oC
δ... BCC crystal with crystal dimension a = 2,93 Angstrom exists at temperature range 1403oC to 1535oC
Solid Phases
The phased diagram includes four solid phases
α Ferrite
..The solid solution of carbon in iron. At 0% C this is pure iron. BCC crystal structure.
The maximum solubility of carbon in iron is 0,02% at 723oC. At 0 oC temperature
the solubility falls to 0,008%. The carbon atoms are located in the crystal interstices.
Austenite
The solid solution of carbon in γ iron is called austenite . This has a FCC crystal structure
with a high solubility for carbon compared with α ferrite. The solubility
reaches a maximum of 2,08% at 1148oC . The solubility decreases to 0,8% at 723 oC
The carbon atoms are dissolved interstitially. The difference in solubility between the
austenite and α Ferrite is the basis for the hardening of steels
Cementite
This is an intermetallic compound which contains 6,67% C and 93,3% Fe. Cementite
is a hard brittle compound with and orthorhombic crystal structure each unit cell has 12 Fe atoms
and 4 C atoms
δ Ferrite..
This is a solid solution of carbon in iron and has a BCC crystal structure. The maximum solubility
or C in Fe is 0,09% at 1495oC. This has no real practical significance in engineering.
Lever Rule
The lever rule can be applied to any phase region an provides an indication
of the proportions of the constituent parts at any point on the phase diagram.
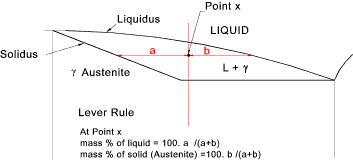
Applying the lever rule to the eutectoid point (0,80% C at 723oC )
Wt% Ferrite = 100 (6,67 -0,8)/ 6,67- 0,02) = 88%
Wt% Cementite = 100 (0,8- 0,02) /6,67- 0,02) = 12%
Steels
If the carbon content of the cooled solid is less than Eutectoid (about 0,8% C) the solid
is identified as a hypoeutectoid steel: most steels are this form. If the carbon content is
more then 0,8% then the solid is a hypereutectoid steel. Hypereutectoid steels with carbon
content over 1,2% C are very brittle. Few steels are made with carbon contents over 1,2%.
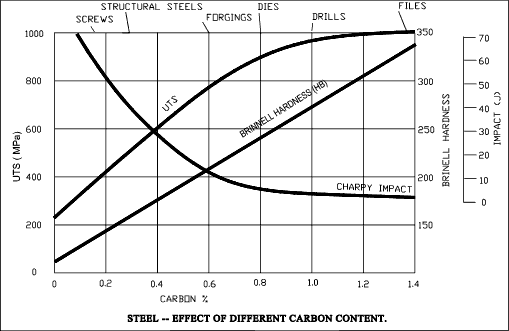
Generally in order to increase the strength of steel other alloying elements are added which increase
the strength while retaining toughness and ductility
A eutectoid solid raised to a temperature just above 723oC and held at this temperature
for some time will become a homogeneous austenite phase. If this solid is then slowly
cooled the entire structure will change to a lamellar structure of alternate plates of ferrite and cementite.
This structure is called pearlite because of its mother-of-pearl appearance. As calculated above
pearlite consists of 88% (mass) ferrite and 12% (mass) cementite
Transformation of a hypereutectoid steel (> 0,8% C) will result in pearlite in a matrix
of cementite. Transformation of a hypoeutectoid steel (< 0,8% C ) will result
in pearlite in a matrix of α Ferrite.
Transformation on high cooling rates
Below is an Isothermal Transformation (IT) diagram, also called a TTT (Time, Temperature, Transformation)
curve for a eutectoid steel test piece which has been rapidly cooled in a bath at a set temperature,
held for a time and then water quenched.
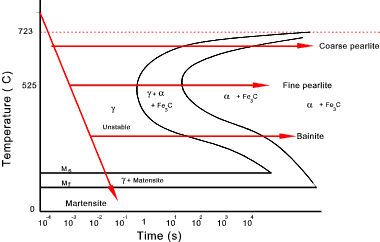
It can be seen that if the transformation is allowed to take place at a higher temperature
then, as above coarse pearlite is formed.
If the test piece is cooled to a lower temperature in a bath a finer
pearlitic structure results.
If the test piece is rapidly cooled to a temperature below a value Ms (which varies
with the carbon content then a new metastable phase is produced called Martensite. Martensite
is a supersaturated solid solution of carbon in ferrite.
If the test piece is cooled rapidly at a temperature between 220oC and 525oC a phase
structure between pearlite and martensite is formed. This is called Bainite
Bainite
Bainite is a constituent which forms from austenite in a tempertures range below 530 oC and above Ms . Bainite forms together
with pearlite in steel which are cooled somewhat too fast to form a complete pearlite
structure. Bainite is like pearlite a mixture of ferrite and iron carbide but in a different
form. The bainite structure varies from a featherlike pattern to pattern of lens shaped particles depending
on the temperature range of formation. (Featherlike constituent in upper temperature range and lens like
in the lower temperature range). Bainite is harder, stronger and tougher than ferrite-pearlite structures at
lower temperatures.
Martensite
Martensite is the hardest structure formed from austenite. It is a distorted
BCC (tetrogonal) it is a body centred tetrogonal structure. The distortion is
caused by trapped carbon atoms which have not been able to nucleate into cementite
Some features of martensite are listed below
- The crystal structure is a stressed structure which is resistance to dislocation movement
it is therefore strong and relatively brittle.
- There are various types of martensite depending on the carbon content:
for C <0,2% the martensite is in the form of well defined thin strips (laths),
for C <= 0,6% plates of martensite are formed ,
for C < = 1,2% the martensite is in the form of arrays of well defined plates
- The martensite phase initiates at a temperature Ms and is complete at a temperature
Mf.
Ms varies from 500oC for C <= 0,1% to 200oC for C <= 1,2%
|
|



