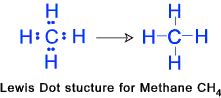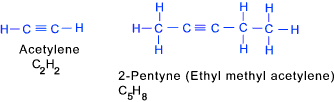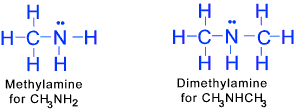Chemistry Index Organic Chemistry
Important Notes
This page is intended to give simple notes on organic chemistry. For detailed
information please consult reputable references and/or use the links provided
at the foot of the page.
The structures I have used are based on lewis dot structures. These
structures do not provide clear 3D geometric information of the molecules and sometimes
may provide misleading information on the relative positions of the bonds.
|
Organic Chemistry The branch of chemistry that is concerned with compounds of carbon.
Therefore this deals with living matter, oil products, plastics etc etc.
Because of the extraordinary bonding properties of carbon there are many more organic compounds than inorganic compounds
The total field of organic chemistry is so large that it is necessary to break it down into sub-groups or families
of compounds .
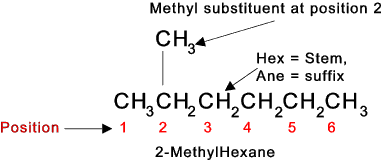
Naming Organic Chemicals The IUPAC (The international Union of Pure and Applied Chemistry) has produced an accepted naming convention (systematic Nomenclature) for chemicals.. The name includes
A simple molecule illustrating the naming convention is shown below
Aliphatic Compounds An aliphatic compound is any chemical compound belonging to the organic class
in which the atoms are not linked together to form a ring. A major structural
groups of organic molecules, the aliphatic compounds include the alkanes,
alkenes, and alkynes, and substances derived from them, by
replacing one or more hydrogen atoms by atoms of other elements or groups of atoms. Alkanes (paraffins) These saturated hydrocarbons with the general formula CnHn+2. All of these substances end with -ane(the IUPAC suffix) and include those listed below. The stem names relate to the number of carbon atoms in the molecules as shown below
etc.etc. Methane (CH4)......
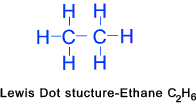
Ethane (C2H6)......
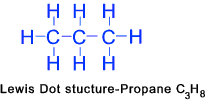
Propane (C3H8)......
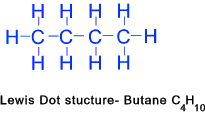
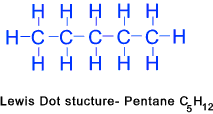
Butane (C4H10)...... Pentane (C5H12)......
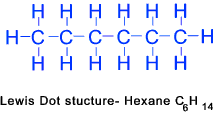
Hexhane (C6H14)......
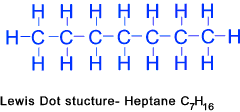
Heptane (C7H16)......
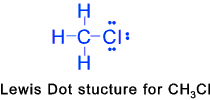
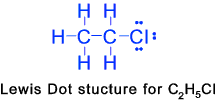
etc. etc. Alkyl Halides (Haloalkanes)
These are organic compounds in which more one or more hydrogen atoms of the alkane series
have been replaced by halogen atoms(atoms with one electron short of stable structure )..Example
are chloromethane CH3Cl, Dibromethane CH2BrCH2Br etc. These
can be formed by direct radiation between the constituent substances with ultravoilet light..
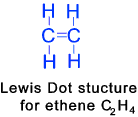
Alkenes
These are unsaturated hydrocarbons that contain at least one double carbon bond.
The general formula for alkenes with only one double bond in the molecule
is CnH2n where n is the number of carbon atoms in the molecule. C2H4 + 3 O2 ==> 2 CO2 + 2 H2O
The first three alkenes are gases, the intermediate alkenes are liquids and higher
members of the olefin series are wax like solids at room temperature.
The alkenes are insoluble in water, but are soluble in organic solvents.
The liquids and solids have a density less than water.
Alkynes These are unsaturated hydrocarbons that contain at least one triple carbon bond.
Lewis structures for two alkynes are shown below..
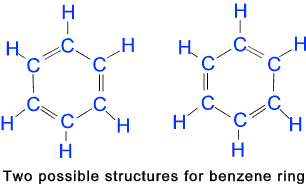
Aromatic Compounds Aromatic Compounds Aromatic compounds are a major class of chemical compounds whose molecular structure
includes one or more planar rings of atoms joined by covalent bonds of two different kinds.
The term aromatic was first used in 1860 for a group of hydrocarbons isolated from
coal tar and characterised by their strong odours. In chemistry, aromaticity has
come to denote the chemical behaviour of this class of molecules.
Alcohols (at least one OH group) All alcohol compounds end with ..ol e.g. methanol, ethanol.
Primary alcohols have two hydrogen atoms on the carbon joined to the -OH groups.
Secondary alcohols have only one hydrogen atom on the carbon joined to the OH group. Tertiary alcohols have
no hydrogen atoms on the carbon attached to the -OH molecule.
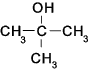
The molecule "2-methylpropan-ol" can be written as shown below..This is a tertiary alcohol
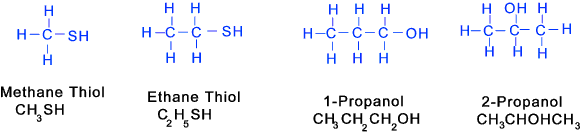
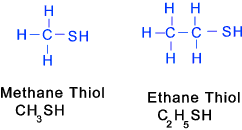
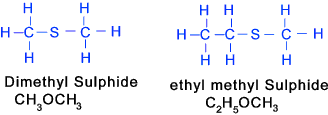
Thiols (similar to alcohols except Sulfur (SH) instead of Oxygen Note: Thio -> sulphur containing
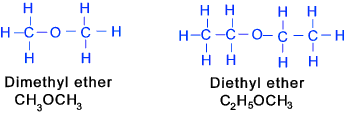
Ethers (at least one Oxygen single bonded to two carbons) These are usually liquids with pleasant odour , but some aromatic and the higher
aliphatic ethers are crystalline solids. They are insoluable
in water but soluble in alcohol and diethyl ether. The commonest ether
diethyl ether is usually simply called ether. Diethyl ether (ethoxyethene) is
an anaesthetic and a very useful organic solvent
Thioethers- Sulphides (similar to ethers except a Sulfur atom in place of an Oxygen atom) Thio 0> Suphur containing..
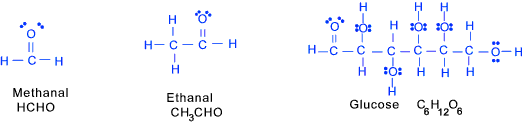
Aldehydes (at least one formyl group -CH=O) These are organic compound containing the group as shown below attached directly onto another carbon atom.
Methanal (formaldahyde) HCHO is a member of this group although it is not a typical aldehyde.
Aledehydes are normally colourless liquids (aliphatic) or solids ((higher aromatics) with characteristic colours.
These compounds are oxidized to acids and reduced to primary alcohols.
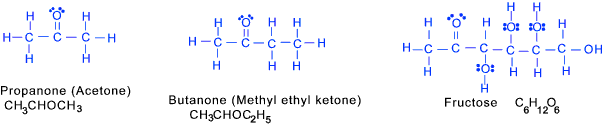
Ketones (at least on keto group C=O)
Chemistry naming conventions always end this range of chemicals with -one
Where Aldehydes have the C=O group (carbonyl) on an end carbon atom Ketones have
it on a middle carbon atom. Secondary alcohols can be oxidixed into ketones.
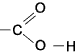
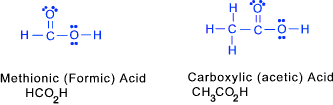
Carboxylic Acids (at least one carboxyl group -COOH)) These include at least one carboxyl group -COOH as shown below
Typical carboxylic acids include methanoic (formic) acid (HCOOH->HCO2H), ethanoic acid-acetic acid (CH3COOH), butanoic acid (CH3CH2CH2COOH ).
Because carboxylic acids have both a lone oxygen and an OH group, they are strongly hydrogen-bonded to each other, therefore having high boiling points.
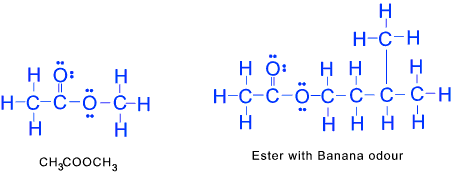
Esters Esters are organic compounds formed by reactions between alcohols and acids . Esters containing simple hydrocarbon groups are volatile fragrant substances used as flavourings in the food industry. In esters one of the oxygens has a double bond the other oxygen atom is bonded to a carbon atom of a hydrocarbon. >/p>
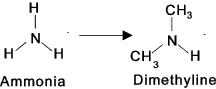
Amines (at least one Nitrogen bonded to Hydrogen or carbon atoms) These organic compounds are produced by replacing one or more of the hydrogen atoms
in ammonia with an organic group.
Amines are classified as primary, secondary, or tertiary depending on whether one,
two, or three of the hydrogen atoms of ammonia have been replaced by organic groups.
Diamines, triamines, and polyamines contain two, three, or more nitrogen groupings of the types identified.
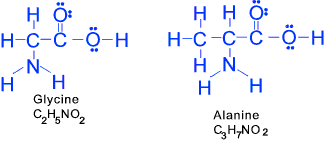
Amino Acids (at least one amino group NH2 and one carboxyl group -COOH) A large group of organic compounds containing both the carboxyl group COOH and the amino group
NH2. These include Glycine H2NCH2COOH. Many proteins
are built up entirely of amino acid groupings by condensation between the NH2 and COOH groups.
Carbohydrates (at least several OH groups and a formyl or keto group)
A group of organic compounds based on the general formula C x(H 2O) y.
The simplest carbohydrates include sugars (saccharides)including glucose and sucrose. Organometallics (with an ionic bonding between a metal and a carbon structure) These are compounds in which metals ions or atoms are bound to organic groups via a carbon to metal bond. These may have
single metal to carbon bonds as in Aluminium Alkyls (Al(CH3)3.
The compounds can also be more complicated double bonds... |
Links relevant to organic chemistry..
|
|