Introduction
This section includes notes on certain important thermodynamic properties including
enthalpy (h), Specific heat (cv,cp),
gas constant (R), and Entropy (S)
Enthalpy
In many thermodynamic fluid process analyses the sum of the internal energy (U) and the
product of pressure (P) and volume(V) is present. The combination (U + PV)
is called the enthalpy of the fluid. H is a thermodynamic fluid property but
is does not have an absolute value(because it includes internal energy U )value and
therefore enthalpy changes are generally applied or enthalpy values are identified
relative to a fixed state e.g. water at 273 deg.K . It is important to note
that enthalpy is simply a combination of properties ..it is not a form of stored energy
although for certain applications it can be treated as energy.
H = U + PV ..........(extensive property)
per unit mass
h = u + Pv ...........(intensive property)
When referring for water and steam and other fluids at different states in tables
the following enthalpy designations are used
- hg..specific enthalpy of saturated vapor
- hf..specific enthalpy of saturated liquid
- hi..specific enthalpy of saturated solid
- hfg..specific latent heat of vaporisation = h g - h f
- hif..specific latent heat of fusion = h f - h i
- hig..specific heat of sublimation = h g - h i
|
Specific Heat Capacity
The heat capacity of a substance is classically defined as the amount of heat needed to raise unit mass of
a substance one degree Centigrade.
In SI units the specific heat capacity is
the amount of heat required to raise 1 kg mass through 1 degree kelvin. (Unit kJ/kg.K)
Note:The specific heat of a substance is the ratio of the heat capacity of a substance
relative to a reference substance generally water.
The heat capacity of water is one calorie per degree C (classical) or (4180 J/kg.K )
The specific heat of a substance relative to water will be numerically
equal to its heat capacity in classical units, but not in SI units ;
The term specific heat is often used when the heat capacity actually is meant. This page
is concerned only with heat capacity (to be called specific heat capacity).
Because the heat capacities of most substances vary with changes in temperature, the
temperatures of both the specified substance and the reference substance must be known
in order to give a precise value for the specific heat.
Specific Heat Capacities of Gases
Four specific heats are for gases are used.
- Cv = Molar specific heat at constant volume.
- Cp = Molar specific heat at constant pressure.
- cv = Specific heat at constant volume.
- cp = Specific heat at constant pressure.
|
Note: The molar specific heats are mainly used for chemical studies
<
The specific heat varies with temperature and pressure. The graph below this illustrates
this characteristic for cp. for air
Tables below show the variation of cp and
cp with temperatures...
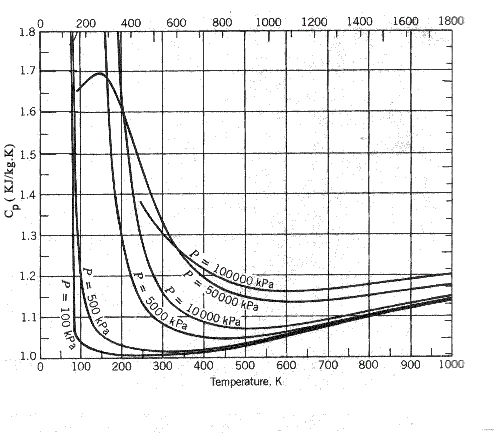
Variation of cp (for Air) with temperature and pressure
Latent Heat
The latent heat of fusion is the amount of heat required to convert unit mass a substance
from solid to liquid without change of temperature..
The latent heat of vaporisation is the amount of heat required to convert unit mass of a
substance from liquid to vapour without change of temperature.
Gas Constant R
The gas constant R is derived from the equation of state
Pv = RT .. for unit mass of gas
PV = mRT
The gas constant R is different for each gas and has different units depending on the
unit systems used. Typical units are (kJ/kg.K).
The universal gas constant Ru is the same for all gases and is defined by
PV = NRuT
- R = Gas Constant = Ru /M
- Ru = Universal Gas Constant
- v = Gas volume (m3 )
- V = Gas Volume (m3 )
- N = Number of Moles
- T = Absolute Temperature deg K
- M = Molar mass (kg)
- P = Absolute Pressure N/m3 (kg)
|
Table of Gas Properties for Various Gases
Based on a pressure of 1.032 bar and at 0oC
| Gas |
cp |
cv |
cp / cv |
cp - cv |
| Acetylene |
1.616 |
1.3 |
1.2431 |
0.316 |
| Air |
1.005 |
0.718 |
1.3997 |
0.287 |
| Ammonia |
2.056 |
1.568 |
1.3112 |
0.488 |
| Argon |
0.52 |
0.312 |
1.6667 |
0.208 |
| Carbon Dioxide |
0.816 |
0.627 |
1.3014 |
0.189 |
| Carbon Disulphide |
0.582 |
0.473 |
1.2304 |
0.109 |
| Carbon Monoxide |
1.038 |
0.741 |
1.4008 |
0.297 |
| Chlorine |
0.473 |
0.36 |
1.3139 |
0.113 |
| Coal Gas |
2.14 |
1.59 |
1.3459 |
0.55 |
| Ethylene |
1.47 |
1.173 |
1.2532 |
0.297 |
| Helium |
5.2 |
3.121 |
1.6661 |
2.079 |
| Hydrochloric Acid |
0.795 |
0.567 |
1.4021 |
0.228 |
| Hydrogen |
14.05 |
9.934 |
1.4143 |
4.116 |
| Hydrogen Sulphide |
0.992 |
0.748 |
1.3262 |
0.244 |
| Krypron |
0.25 |
0.151 |
1.6556 |
0.099 |
| Methane |
2.19 |
1.672 |
1.3098 |
0.518 |
| Neon |
1.03 |
0.618 |
1.6667 |
0.412 |
| Nitrogen |
1.038 |
0.741 |
1.4008 |
0.297 |
| Oxygen |
0.909 |
0.649 |
1.4006 |
0.26 |
| Propane |
1.549 |
1.36 |
1.1390 |
0.189 |
| Sulphur Dioxide |
0.586 |
0.456 |
1.2851 |
0.13 |
| Water Vapor |
1.842 |
1.381 |
1.3338 |
0.461 |
| Xenon |
0.16 |
0.097 |
1.6495 |
0.063 |
Zero Pressure - (pseudo ideal gas) Gas properties - Showing Temperature relationships
Air
| Temperature |
cp |
cv |
cp / cv |
cp - cv |
| 0 |
1.004 |
0.717 |
1.4003 |
0.287 |
| 50 |
1.006 |
0.719 |
1.3992 |
0.287 |
| 100 |
1.01 |
0.723 |
1.3970 |
0.287 |
| 150 |
1.016 |
0.729 |
1.3937 |
0.287 |
| 200 |
1.024 |
0.737 |
1.3894 |
0.287 |
| 400 |
1.068 |
0.781 |
1.3675 |
0.287 |
| 600 |
1.115 |
0.828 |
1.3466 |
0.287 |
| 800 |
1.154 |
0.867 |
1.3310 |
0.287 |
| 1000 |
1.185 |
0.898 |
1.3196 |
0.287 |
| 1500 |
1.235 |
0.948 |
1.3027 |
0.287 |
| 2000 |
1.266 |
0.978 |
1.2945 |
0.288 |
| 2500 |
1.287 |
1 |
1.287 |
0.287 |
|
Carbon Dioxide
| Temperature |
cp |
cv |
cp / cv |
cp - cv |
| 0 |
0.817 |
0.628 |
1.3010 |
0.189 |
| 50 |
0.869 |
0.68 |
1.2779 |
0.189 |
| 100 |
0.916 |
0.727 |
1.2600 |
0.189 |
| 150 |
0.958 |
0.769 |
1.2458 |
0.189 |
| 200 |
0.995 |
0.806 |
1.2345 |
0.189 |
| 400 |
1.113 |
0.924 |
1.2045 |
0.189 |
| 600 |
1.195 |
1.006 |
1.1880 |
0.189 |
| 800 |
1.253 |
1.064 |
1.1776 |
0.189 |
| 1000 |
1.294 |
1.105 |
1.1710 |
0.189 |
| 1500 |
1.354 |
1.165 |
1.1622 |
0.189 |
| 2000 |
1.387 |
1.198 |
1.1578 |
0.189 |
| 2500 |
1.407 |
1.218 |
1.1552 |
0.189 |
|
Carbon Monoxide
| Temperature |
cp |
cv |
cp / cv |
cp - cv |
| 0 |
1.04 |
0.743 |
1.3997 |
0.297 |
| 50 |
1.041 |
0.745 |
1.3973 |
0.296 |
| 100 |
1.045 |
0.748 |
1.3971 |
0.297 |
| 150 |
1.05 |
0.754 |
1.3926 |
0.296 |
| 200 |
1.074 |
0.777 |
1.3822 |
0.297 |
| 400 |
1.106 |
0.809 |
1.3671 |
0.297 |
| 600 |
1.157 |
0.86 |
1.3453 |
0.297 |
| 800 |
1.199 |
0.902 |
1.3293 |
0.297 |
| 1000 |
1.231 |
0.934 |
1.3180 |
0.297 |
| 1500 |
1.28 |
0.983 |
1.3021 |
0.297 |
| 2000 |
1.306 |
1.01 |
1.2931 |
0.296 |
| 2500 |
1.323 |
1.026 |
1.2895 |
0.297 |
|
Hydrogen
| Temperature |
cp |
cv |
cp / cv |
cp - cv |
| 0 |
14.19 |
10.07 |
1.4091 |
4.12 |
| 50 |
14.37 |
10.25 |
1.402 |
4.12 |
| 100 |
14.46 |
10.33 |
1.3998 |
4.13 |
| 150 |
14.49 |
10.37 |
1.3973 |
4.12 |
| 200 |
14.51 |
10.38 |
1.3979 |
4.13 |
| 400 |
14.59 |
10.46 |
1.3948 |
4.13 |
| 600 |
14.79 |
10.66 |
1.3874 |
4.13 |
| 800 |
15.12 |
10.99 |
1.3758 |
4.13 |
| 1000 |
15.53 |
11.41 |
1.3611 |
4.12 |
| 1500 |
16.58 |
12.46 |
1.3307 |
4.12 |
| 2000 |
17.45 |
13.33 |
1.3091 |
4.12 |
| 2500 |
18.12 |
14 |
1.2943 |
4.12 |
|
Nitrogen
| Temperature |
cp |
cv |
cp / cv |
cp - cv |
| 0 |
1.039 |
0.742 |
1.4003 |
0.297 |
| 50 |
1.04 |
0.743 |
1.3997 |
0.297 |
| 100 |
1.042 |
0.745 |
1.3987 |
0.297 |
| 150 |
1.046 |
0.749 |
1.3965 |
0.297 |
| 200 |
1.052 |
0.755 |
1.3934 |
0.297 |
| 400 |
1.091 |
0.795 |
1.3723 |
0.296 |
| 600 |
1.139 |
0.842 |
1.3527 |
0.297 |
| 800 |
1.181 |
0.885 |
1.3345 |
0.296 |
| 1000 |
1.215 |
0.918 |
1.3235 |
0.297 |
| 1500 |
1.269 |
0.972 |
1.3056 |
0.297 |
| 2000 |
1.298 |
1.001 |
1.2967 |
0.297 |
| 2500 |
1.316 |
1.019 |
1.2915 |
0.297 |
|
Oxygen
| Temperature |
cp |
cv |
cp / cv |
cp - cv |
| 0 |
0.915 |
0.655 |
1.3969 |
0.26 |
| 50 |
0.922 |
0.663 |
1.3906 |
0.259 |
| 100 |
0.934 |
0.674 |
1.3858 |
0.26 |
| 150 |
0.948 |
0.688 |
1.3779 |
0.26 |
| 200 |
0.963 |
0.703 |
1.3698 |
0.26 |
| 400 |
1.024 |
0.764 |
1.3403 |
0.26 |
| 600 |
1.069 |
0.809 |
1.3214 |
0.26 |
| 800 |
1.1 |
0.84 |
1.3095 |
0.26 |
| 1000 |
1.122 |
0.863 |
1.3001 |
0.259 |
| 1500 |
1.164 |
0.904 |
1.2876 |
0.26 |
| 2000 |
1.2 |
0.94 |
1.2766 |
0.26 |
| 2500 |
1.234 |
0.975 |
1.2656 |
0.259 |
|
| 