Thermodynamics & Heat Transfer
This page provides a limited notes on thermodyamics and heat transfer that may be useful to mechanical engineers.
Definition
Thermodynamics ..From the Greek thermos meaning heat and dynamis meaning power.
Thermodynamics covers the relationship between heat, work, temperature, and energy, including the general behaviour of physical systems in a condition of equilibrium or close to it. It is a fundamental part of all the physical sciences.
Thermodynamics is an experimental science based on a small number of principles that are derived from experience. Classical Thermodynamics is concerned only with macroscopic or large-scale properties of matter and does not include the study of small-scale or microscopic structure of matter. Statistical Thermodynamics ( not covered on this website) includes the study of the thermal relationships of atomic sized particles
Notation
| Identifier | Description | Units (typical) |
| c p | Specific Heat Capacity at Constant pressure | kJ/(kg K) |
| c v | Specific Heat Capacity at Constant Volume | kJ/(kg K) |
| p | Absolute Pressure | N / m 2 |
| T | Absolute Temperature | K |
| v | volume per unit mass | m 3 |
| W | Work Output per unit mass | kJ/kg |
| M | Molecular Weight | - |
| R o | Universal Gas Constant = 8,31 | kJ /(kg mole.K) |
| Q | Heat Quantity | kJ |
| R | Gas Constant = R o / M | kJ /kg.K |
| U | Internal energy (thermal) | kJ |
Thermodynamic Laws
Zeroth Law of Thermodynamics
When two objects are separately in thermodynamic equilibrium with a third they are in equilibrium with each otherObjects in thermodynamic equilibrium are at the same temperature
First Law of Thermodynamics...
This law expresses the general law of conservation of energy. and states that heat and work are mutually convertible
Heat In = Work Out over complete cycle
or Sum (d Q ) = sum (d W )
dQ = dU + dW
Second Law of Thermodynamics
This law in its simplest states that heat can only flow from hot to cold and not vice versa. In terms of thermodynamic engine cycles the law states that the gross heat supplied to a system in a complete cycle must exceed the work done by the system. Therefore heat must be rejected. The thermal efficiency of an heat engine must be less than 100%.Process Relations
Reversible Polytropic Process
p v n = constant
W = ( p 2 v 2 - p 1 v 1 ) / ( 1 - n ) .. (n not 0 )
For a perfect gas
W = R ( T 2 - T 1 ) / (1 -n )
Q = ( Cv + R /(1 - n) ) ( T 2 -T 1 )
T 2 / T 1 = ( p 2 / p 1 ) ( n-1 ) / n
For Adiabatic processes (Q = 0 ) n = γ = cp / cv
γ = 1.4 for Air, H 2, O 2, CO, NO, Hcl
γ = 1.3 for CO 2, SO 2, H 2O, H 2S, N 2O, NH 3, CL 2, CH 4, C 2H 2, C 2H 4
Heat Transfer
Heat Transfer takes place by Conduction, Convection and Radiation
Heat Transfer by Conduction
- q = Heat Flow Rate W
- t 1 & t 2 = temperature, K (heat flows down (-))
- A = Area, m 2
- k = Coefficient of thermal conductivity, W m -1K -1
- U = Overall Heat Transfer Coefficient,W m -1K -1
- h = Surface Heat Transfer Coefficient, W m -2K -1
- R = Thermal Resistance, W -1.m -1.K
| dq = kA(-dt/dx) |
| q = (k.A /x). (t 1-t 2) |
| U = k/x |
| Therefore q = U.A(t 1-t 2) |
| Thermal resistance R = 1 / U.A |
The heat has to pass through the surface layers on both sides of the wall
| q = A.h s1(t s1 - t 1) =
k.A(t 1 -t 2) / x =
Ah s2(t 2 -t s2) |
| U = 1 / (1/h s1 + x/k + 1/h s2 ) |
| R = 1/A.h s1 + 1/A.h s2 + x/A.k = R s1 + R s2 + R |
Table Showing Various values for k at 20 oC
|
|
|
Heat Transfer by Radiation
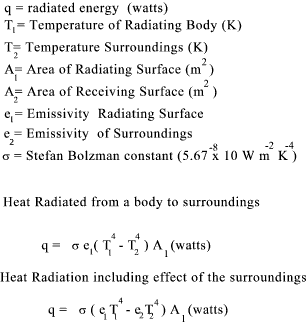
Emissivity Values
Refer to link Emissivity Values for better table
| Surface Material | Emmissity | Surface Material | Emmissity |
|---|---|---|---|
| Aluminium-Oxidised | 0.11 | Tile | 0.97 |
| Aluminium-Polished | 0.05 | Water | 0.95 |
| Aluminium anodised | 0.77 | Wood-Oak | 0.9 |
| Aluminium rough | 0.07 | Paint | 0.96 |
| Asbestos Board | 0.94 | Paper | 0.93 |
| Black Body -Matt | 1.00 | Plastics | 0.91 Av |
| Brass -Dull | 0.22 | Rubber-Nat_Hard | 0.91 |
| Brass- Polished | 0.03 | Rubber _Nat_Soft | 0.86 |
| Brick -Dark | 0.9 | Steel_Oxidised | 0.79 |
| Concrete | 0.85 | Steel Polished | 0.07 |
| Copper-Oxidised | 0.87 | St.Steel-Weathered | 0.85 |
| Copper -Polished | 0.04 | St.Steel-Polished | 0.15 |
| Glass | 0.92 | Steel Galv. Old | 0.88 |
| Plaster | 0.98 | Steel Galv new | 0.23 |
Heat Transfer by Convection
Convective heat transfer occurs between a moving fluid and a solid surface.The rate of convective heat transfer between a surface and a fluid is given by the Newton�s Law of Cooling;
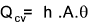
It is customary to express the convection coefficient (average or local), in a non-dimensional form called the Nusselt Number.
Natural convectionNu = C(Gr.Pr) n C and n are tabled below
Note: Convection heat transfer values are very specific to the geometry of the surface and the heat transfer conditions - These example equations are very general in nature and should not be used for serious calcs. The links below provide much safer equations..
| Surface | (Gr.Pr) | C | n |
| Vertical Plates/Cylinders | 10 4 to 10 9 | 0.59 | 0.25 |
| 10 9 to 10 12 | 0.13 | 0.33 | |
| Horizontal Pipes | 10 3 to 10 9 | 0.53 | 0.25 |
| Horizontal Plates Heated Face up or Cooled Face Down | 10 5 to 2 x 10 7 | 0.54 | 0.25 |
| 2 x10 7 to 3 x10 10 | 0.14 | 0.33 | |
| Horizontal Plates Heated Face up or Cooled Face Down | 3 x10 5 to 3 x10 10 | 0.27 | 0.25 |
Forced Convection
Laminar flow over Plate Nu = 0.664(Re) 1/2(Pr) 1/3
Fully Developed pipe flow Nu = 0.0866(D/L)Re.Pr / (1+0.04[D / L(Re.Pr)] 2/3) + 3.66
Turbulent Flow Over Flat Plate Nu = 0.036Pr 1/3Re 0.8
Turbulent Flow In Pipe Nu = 0.023Pr 0.4Re 0.8
Typical Values of Heat Transfer Coefficient h = W.m -2K -1
- Free Convection Over Various Shape - Air 2 - 23
- Free Convection Over Various Shape - Water 300 - 1700
- Turbulent Convection Over Various Shape and through tubes - Air 6 - 1400
- Turbulent Convection Over Various Shape and through tubes - Water 1100 - 9000
Heat Exchangers
Heat exchangers normally transfer energy from a hot fluid to a colder fluid.
The energy in = The energy out.
If the fluids are the same with the same specific heat. The mass flowrate x the temp drop of the hot fluid = the mass flow rate
x the temp rise of the cold fluid.
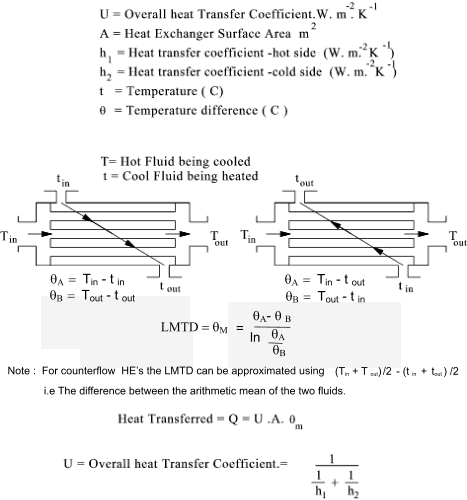
Typical Values for Overall Heat transfer U are
- Plate Heat Exchanger, liquid to liquid U range 1000 > 4000 W. m.-2K.-1
- Shell and Tube, liquid inside and outside tubes U range150 > 1200 W. m.-2K.-1.
- Spiral Heat Exchanger, liquid to liquid U range 700 > 2500 W. m.-2K.-1
In this section
- Thermal Insulation
- Thermal Insulation Tables
- Thermodynamics
- Thermodynamics
- Thermodynamics Air compressors & motors
- Thermodynamics Boilers
- Thermodynamics Carnot Cycle
- Thermodynamics Combustion
- Thermodynamics Condensers & Cooling Towers
- Thermodynamics Entropy
- Thermodynamics First Law Non Flow examples
- Thermodynamics Fundamentals
- Thermodynamics Heat Transfer
- Thermodynamics Laws
- Thermodynamics Properties
- Thermodynamics Rankine cycle Superheated
- Thermodynamics Steam Flow
- Thermodynamics Steam Tables
- Thermodynamics Steam Tables
- Thermodynamics Steam Tables Index
- Thermodynamics Steam Tables Superheated Steam
- Thermodynamics Steam Tables Superheated Steam
- Thermodynamics Steam Tables Superheated Steam
- Thermodynamics Steam Tables Superheated Steam
- Thermodynamics Steam Turbine
- Thermodynamics Two / Four Stroke Engine Notes