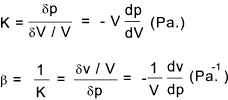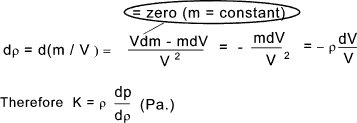| |
Fluids Index Fluid Characteristics /Properties...
Introduction..... Symbols..... Density..... Pressure..... Viscosity..... Surface Tension..... Compressibility..... Sonic Velocity (Mach No).....
|
Introduction.... Thermodynamic Properties Fluids can be either liquids or gases. A liquid is hard to
compress and takes the shape of the vessel containing it. However it has a fixed volume
and has an upper level surface. Gas is easy to compress, and expands to fill
its container. There is thus no free surface. Liquids are generally assumed to
be incompressible fluids and gases compressible fluids. Liquids ar
only compressible when they are highly pressurised, and the compressibility of gases may be
disregarded whenever the change in pressure is very small. Symbols / Units
Density
The mass per unit volume of material is called the density, which is
generally expressed by the symbol ρ. The density of a gas changes according
to the pressure, but that of a liquid may be considered constant unless the relevant pressures changes are very high The units of density are kg/m3 (SI).
Note: The for liquids and solids the specific gravity is generally identified as the ratio of the materials density relative to water both at the same conditions i.e 4o and 1,013 bara ambient pressure. For gases the specific gravity is the ratio of the materials density to the the density of air, both at the same conditions as identified above. The term specific gravity is not as clear at term as the relative density. For a liquid RD = ρ /ρw The apparent specific gravity sga is the ratio of the weight of a volume of a material to the weight of an equal volume of the reference fluid (water or air) again under the same conditions. This is a practical value but essentially is the same as the true specific gravity. The density of gases gases and vapours are greatly affected by the pressure . For so called perfect gases the density can be calculated from the formula .
Ro = the universal Gas constant = 8314 J/(kg.K) and M = Molecular weight.
Therefore R = 8314/M [ J/(kg.K) ] The reciprocal of density, i.e. the volume per unit mass, is called the specific volume, which is generally expressed by the symbol υ υ = 1/ρ The dimensional formula for density = ML-3 and the dimensional formula for specific volume = M-1L3 Pressure A fluid is always subject to pressure. Pressure is the force
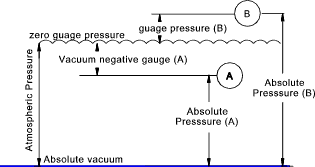
per unit area at a point. The absence of pressure occurs in a complete
vacuum. A complete vacuum is really a theoretical concept.
pabs = pgauge + patm The SI unit of pressure is the Pascal (abrev.= Pa) (Newton /m2 ). The dimensional formula for pressure
is ML-1T-2.
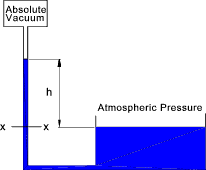
The liquid will be forced up the tube until the gravity force resulting from the level of fluid in the tube balances the force due to the pressure at the bottom of the head of fluid. Assuming the area of the tube is A t, the density of the fluid = ρ,and the pressure at the top of the tube is zero. The force at x-x Fxx = 0 + hAρg. The pressure at x-x= Pxx = 0 + hAρg. / A = hρg. For a fluid with a known fixed density the height h can be conveniently used to identify the pressure.
For water the atmospheric pressure is about 10,5m. In practice water vaporises into the vacuum at the top of
the tube reducing the vacuum this reduces the column height by about 180mm.
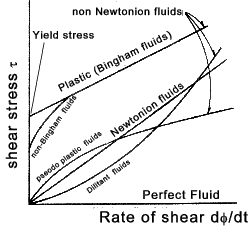
Viscosity... Additional ref notes.. Viscosity
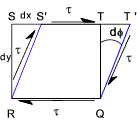
Consider an element of fluid STQR which is subject to a shear stress τ
In a short period of time dt the fluid element distorts to S'T'QR. The fluid will experience a strain φ in time dt. μ ( dφ /dt ) = μ τ Note: The rate of shear strain is also measured as the deflection dx divided by the distance dy i.e dx/dy occuring over a time intervel dt. It is is effectively the velocity gradient dv /dy .. (dv = dx/dt) = μ (dv/dy) = τ .... therefore ..... μ = τ / (dv/dy) If the element where an elastic solid it would distort a fixed amount proportional to the shear stress and the proportionality
constant is called the Modulus of Rigidity (G). The fluid element distorts
at a rate based on the viscosity of the fluid.
Typical Non-Newtonion fluids include. Pseudo plastic fluids e.g. solutions including gelatine, clay, milk and blood
often have reduced viscosity when the rate of shear is increased. Surface Tension The surface of a liquid is the interface between the liquid volume
and the fluid above the liquid. Generally the liquid is water and the fluid
above the liquid is air. The molecules within the liquid attract each other and
at the interface there are more attractive forces towards the bulk of the liquid than
there are towards the adjacent gas molecules. The molecular forces tend
to pull into the fluid bulk. The surface of a liquid is apt to shrink,
and its free surface is in such a state that each section pulls another as if an
elastic film is being stretched. The surface behaves like a flexible membrane.
This property is evident when overfilling a cup with water. The level of
water in the cup will be higher than the cup edge before it overflows.
For large volumes of liquid the forces due to gravity and inertia are
large compared to the surface tension forces. Therefore the surface tension is not considered in most hydrostatic and hydrodynamic
calculations. Compressibility The volume of a fluid changes from V to V + δV as a result of the applied pressure changing from p to p + δp. The compressibility (β) is basically (δV / V ) /δp i.e. the ratio of the proportional change of volume to change of pressure . This is the reciprocal of the bulk modulus K. The bulk modulus K is similar to the spring factor , that is K .(δV/V) = δp
The volume of the fluid clearly decreases if the pressure increases and is proportionate assumed
that the fluid does not change state during the process (it remains a liquid, solid or gas.
For gases the bulk modulus is very much dependent on the conditions : if the compression takes
place at constant temperature the bulk modulus is called the isothermal bulk modulus and if the
compression takes place with no transfer of heat across the system boundary the bulk modulus is colled
the isentropic bulk modulus. The ratio of isentropic/isothermal bulk modulii is γ which is
the ratio of specific heats. Speed of Sound The propagation speeds of traveling waves are characteristic of the media in which they travel and are generally not dependent upon the other wave characteristics such as frequency, period, and amplitude. The speed of sound in air and other gases, liquids, and solids is predictable from their density and elastic properties(bulk modulus). In a fluid medium the wave speed takes the general form
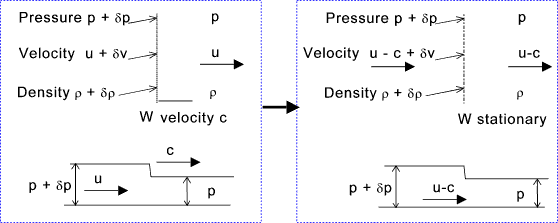
Consider a fluid in which a sound wave is being transmitted at a velocity c. The fluid velocity is u. To simplify the assessment this has been resolves such that the wave is stationary and the fluid has a velocity u-c. See figure below.
Taking a small area normal to the wave front ΔA continuity requires that
For the volume enclosed by ΔA the force to the right = (p + δ p)ΔA -pΔA Elimination of δ u from (A) and (B) above
For a weak pressure wave with δp and δρ --> zero
This equation states that a sound wave which is a weak pressure surge of
value √(∂ p /∂ ρ )
move through a fluid at a velocity of
Considering gases subject to isentropic processes. The law pvγ = constant (k) applies. Therefore
The bulk modulus as defined above K = - v.(dp/dv) and therefore K = γp and therefore for a perfect isentropic gas
The mach number M is the ratio of the velocity of gaseous flow in relation to the sonic velocity
Fluids velocities less than the speed of sound are called sub-sonic (M < 1) and fluid velocities greater than the speed of sound are called supersonic (M >1 ) Table showing approximate sonic velocities for various mediums
|
Useful Links
|
|