Thermos Index Thermodynamics Fundamentals
Work.....
Energy.....
Heat.....
System.....
Boundary.....
Surroundings.....
State.....
Property.....
Process.....
Reversible / Irreversible processes.....
Ideal Gas.....
Perfect Gas.....
Semi Perfect Gas.....
Thermal Efficiency.....
|
Introduction This page identifies the factors that are fundamental to understanding thermodynamics. Work Work is defined simply as the product of force (SI units = newtons) and the distance moved in the direction of the force (SI units = metres). If a block is moved against a constant frictional force of 1 N through a distance of 1 m then 1 N.m (= 1 Joule), of work has been exerted in moving the block..... Energy
Energy at is simplest level is the capacity of a system to do work.
A compressed spring has potential energy because, when released it can exert,
a force over a distance until the spring has is no longer compressed.
The total stored energy of a body, substance or system is a property and is given the symbol E and is generally used not as an absolute value but as measure of change between different states ΔE. The change of internal energy can be obtained from measurements of heat and work.
The stored energy of a body, substance or system which is independent of electricity, atomic, sound, magnetism, surface tension, motion and gravity is called the internal energy is given the symbol U.
Notes on the internal energy and the total stored energy and its relevance to the first
law are provided on webpage Thermodynamic Laws Heat Heat is the energy form which is transferred by a difference in temperature.
If a body at 50 deg C is positioned in a fluid at 10 deg C. The fluid temperature
rises as heat is transferred from the body. (At the microscopic level the energy possessed as a result
of its temperature is kinetic energy of the molecules and atoms.)
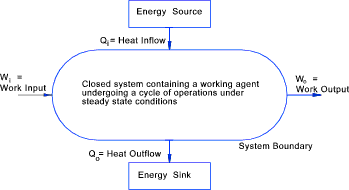
System In thermodynamics a system is a 3D region in space under study. A system can be an isolated, adiabetic, closed system or an open system and it is surrounded by
a defined boundary.. The outside of the boundary is called the surroundings
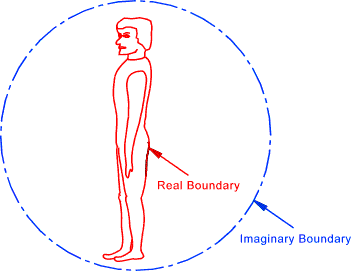
Boundary The boundary encloses a system and may be real (physical) or imaginary.
Control Surface An open system is often called a control volume and its boundary and most specifically
the boundary under scrutiny is called the control surface. Mass may flow across
a control surface. A cylinder piston in motion may be a control surface.
Surroundings Everything outside a system boundary is called the surroundings. Normally the term surroundings is restricted to those outside the system that in some way interact with the system or affect the behaviour of the system. Thermodynamic State of a System The condition of the system as characterized by the values of its
state properties.
Property ..A characteristic of a system In the context of a thermodynamic system a
"state property" identifies a condition of the system (or substance) at an equilibrium
state which is independent of the path of the process by which the state has
been reached. The change in the pressure (P) or the temperature (T) of a
system between two equilibrium states is the same for all paths and therefore the
pressure and temperature are properties. All properties of equilibrium
states e.g. P,V,m and T are characteristics of the activities of large quantities
of molecules..
Some Typical Thermodynamic (intensive) properties of fluids include..
A primitive property can in principle be specified by describing an
simple operation or test to which the system is subjected.e.g using mechanical
measurements ... pressure, volume, thermometric temperature (T) and heat
capacity. Process A thermodynamic process may be defined as the progress of a thermodynamic
system proceeding from an initial state to a final state.
The series of states the substance or system experiences as it progresses through
the process is called the path of the process.
There are a number of thermodynamic process types encountered by engineers including non-flow, steady flow, semi-flow and unsteady flow. These are described as follows:
Reversible /Irreversible Process
If the substance or system passes through a continuous series of equilibrium states
in progressing through the process as it receives or rejects energy it is referred
to as a reversible process. The path of this theoretical process is generally
shown on diagrams as a full line. If the process is reversed, in the thermodynamic sense,
it would leave no trace of itself. Ideal Gas An ideal gas is one that behaves according according to the assumptions that the volume of a gas molecules can be discounted and that molecules do not attract each other. Various relationships have been arrived at for and ideal gas including Charles Law, Boyle's Law and Avogradros Law. ref. Ideal Gas .. An ideal gas conforms to the ideal gas law Pv = nRT
The laws and rules for ideal gases are only reasonably accurate for gases at low pressures and moderately high temperatures...At pressures around 1 bara or less the ideal gases are generally reasonably accurate for real gases.
Perfect Gas
A perfect gas is an ideal gas for which the values of the specific heats
cp and cv are assumed constant.
ref. Properties
This is an idealisation of the behaviour of real gases at low pressures e.g. oxygen
and nitrogen at atmospheric pressure and ambient temperature. Semi-Perfect Gas Semi perfect gases are those subject to a wide variation of temperature such that it cannot be assumed that the specific heats are constan. Semi Perfect gases are ideal gases for which the values of the specific heats cp and cv are allowed to vary as function of T alone. ref. Properties. Thermal Efficiency
The thermal efficiency is a measure of the success of a thermodynamic process.
It is generally expressed in simple terms as the ratio of the energy desired and the energy expended.
Typical efficiencies include
|
Relevant Links
|
|
Thermos Index