Thermos Index First Law of Thermodynamics
Non-Flow Processes
|
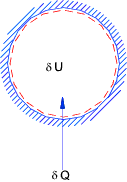
Introduction The intrinsic internal energy U is the total internal energy minus the energies of motion, gravitational, magnetic and surface forces energies . The first law can be written using U as δQ = δU + δW This is termed the restricted energy conservation equation for a system The notes below relate to
various non flow processes using the restricted energy conservation equation when the
kinetic and potential and surface energies are assumed zero. Heating at constant volume
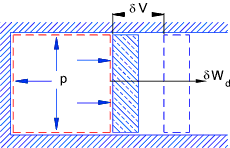
Adiabetic expansion in a cylinder
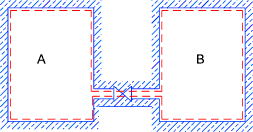
Free Expansion (Joules experiment)
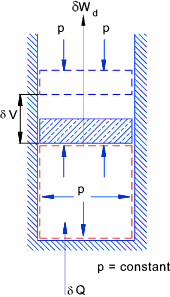
Initially valve is closed and pA > pB > Heating at constant pressure
Note: |
Thermodynamic Law Links
|
|
Thermodynamic Laws