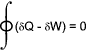Thermos Index Laws of Thermodynamics
|
Introduction The three laws of thermodynamics as noted below are very simple relatively obvious statements based on observations of the laws of nature. They are however of fundamental importance to all physical and chemical processes. Zeroth Law of Thermodynamics... When two objects are separately in thermodynamic equilibrium with a third they are
in equilibrium with each other. First Law of Thermodynamics... This law expresses the general law of conservation of energy. and states that heat and work are mutually convertible Heat In = Work Out over complete cycle
In a cyclic process any property of the system are the same at the end of a cycle as at the beginning. Throughout the path of a cycle (δQ - δW) represents a change in the total stored internal energy property of the system δE . The basic energy equation results from this δQ = δE + δW The total stored internal energy E includes for various forms of energy including
Note: In classical thermodynamics as applicable to mechanical engineering the atomic energy and the chemical energy are not considered.... E = U + P.E + K.E + S.E P.E = total potential energy, K.E = total kinetic energy, S.E = total surface energy . The intrinsic internal energy U is the total internal energy minus the energies of motion, gravitational, magnetic and surface forces energies . The first law can be written using U as δQ = δU + δW This is termed the restricted energy conservation equation for a system. U is
dependent on temperature and is not dependent
on pressure or volume.
Examples of various non flow processes using the restricted energy conservation equation Non-Flow Processes Second Law of Thermodynamics... This law is derived from the whole field of physical experience. Although it is not possible to prove
the law or deduce it from other laws , no exception to it has yet been found.
Some simple conclusions resulting from this law are..
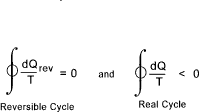
The Clausius inequality is a corollary of the second law and states that if a system completes a cycle
then.
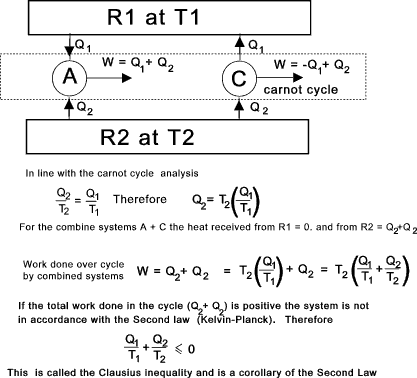
This principle can be tested by considering a system A which completes a complete cycle
receiving heat Q1 from heat source R1 and heat Q2 from source R2. In accordance with the first law the work done by A over
a complete cycle = Q1 + Q2. This system can now be isolated from the heat sources.
A second system C is provided which then completes a Carnot cycle which is
arranged to reject Q1 to the heat source R1 and receive heat Q2from R2. The carnot engine
can be adjusted to match the conditions by setting of the isotherms and if necessary by having multiple cycles
The value (dQ/T ) is a measure of the value of a property of the system called the entropy. Entropy is defined as
Notes on entropy are found on webpage Entropy |
Thermodynamic Law Links
|
|
Thermos Index