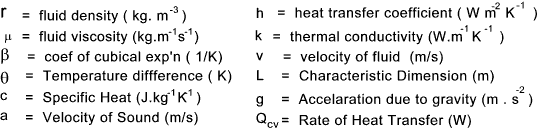Thermos Index Heat Transfer
Introduction..... Symbols..... Heat transfer by conduction..... Heat transfer by radiation..... Heat transfer by Convection..... Heat Exchangers.....
|
Introduction This page provides notes on heat transfer that may be useful
to mechanical engineers. The subject is very complicated and any user who requires
accurate heat transfer values is advised to refer to quality reference documents or use
specialised software.
Conduction Conduction takes place in solids, liquids, and gases. Solids offer
the least resistance to transfer of heat by conduction. Conduction requires physical
contact between material through which the heat is transferred. A materials temperature
is related to the motion of the constituent molecules. The conduction process involves the
molecule moving at higher velocities transferring their kinetic energy to the adjacent molecures
which have lower kinetic energy. Symbols
Heat Transfer by Conduction
The heat has to pass through the surface layers on both sides of the wall
Q r = radiated energy (W) Heat radiation from a body to the surroundings Q r = α e1 (T14 - T24 ) A1 Heat radiation including the effect of the surroundings Q r = α ( e1 T14 - e2T24 ) A1 Now the heat transfer using the heat transfer coefficient = Q r = h r A 1 ( T 1 - T 2 )
therefore h r = α e 1 (T 1 + T 2 )( T 12 + T 22 ) Refer to link Emissivity Values
for better table Convective heat transfer occurs between a moving fluid and
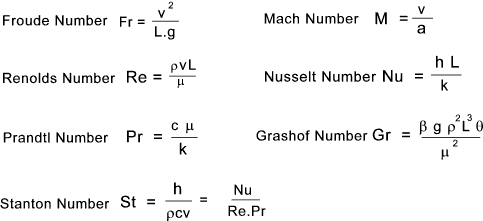
a solid surface. The symbols involved in convective heat transfer are listed below The dimensionless groups involve in convective heat transfer are listed below It is customary to express the convection coefficient (average or local),
in a non-dimensional form called the Nusselt Number. Nu = C(Gr.Pr) n C and n are tabled below Note: Convection heat transfer values are very specific to the geometry of the surface
and the heat transfer conditions - These example equations are very general in nature
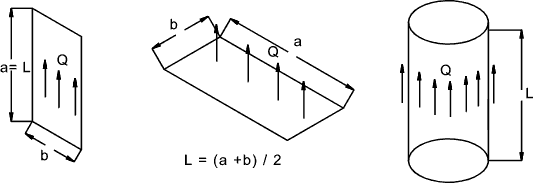
and should not be used for serious calcs. The links below provide much safer equations.. Forced Convection Laminar flow over Plate Nu = 0,664(Re) 1/2(Pr) 1/3 Fully Developed pipe flow Nu = 3,66 + 0,0866(D/L)Re.Pr / (1+0.04[D / L(Re.Pr)] 2/3) Turbulent Flow Over Flat Plate Nu = 0,036Pr 1/3Re 0.8 Turbulent Flow In Pipe Nu = 0,023Pr 0.4Re 0.8 D = Diameter, L = Length, mean film temperature properties assumed Typical Values of Heat Transfer Coefficient h = W.m -2K -1
Heat Exchangers Heat exchangers normally transfer energy from a hot fluid to a colder fluid.
The energy in = The energy out. 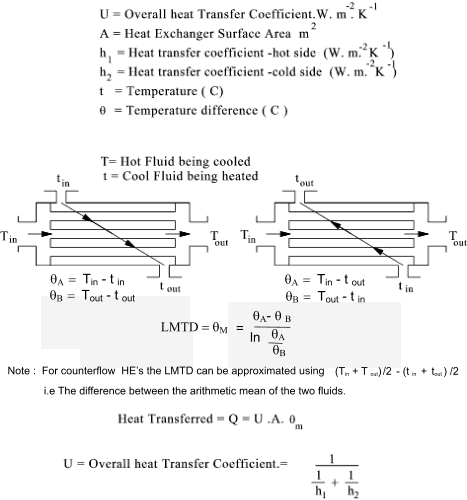 Typical Values for Overall Heat transfer U are
|
Thermodynamic /Heat Transfer Links
|
|
Thermos Index