Introduction
The pages linked provide steam table data including temperature-pressure referenced specific volume,
internal energy, enthalpy and entropy values. The information is listed in SI units
A typical plot which can be produced using the tables is shown below for the T vs h. Similar plots
could be produced for v. s. and u. The critical point C at which the
there is no difference between the liquid and gaseous phase is at 221.2 bar and 374.15 oC.
The Triple point at which solid ,liquid and gas phases can all exist together is at 0.0061 bar and 0.01 oC.
This triple point is used as the zero datum for specific enthalpy, entropy, and internal energy.
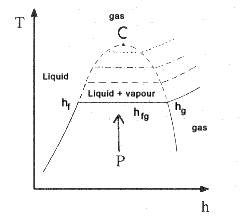
Note: As stated above 0.1o C is the datum (zero) point for enthalpy, entropy, and internal energy
values
Notation
- P = Absolute pressur (bar) - conditions in steam tables are directly related to the pressure
- t s = Saturation temperature (boiling point) in deg Celsius at pressure P
- v f = Specific volume of saturated liquid (No gas content but at the temperature of evaporation)..m3/kg
- v g = Specific Volume of dry saturated gas (No liquid content-but no superheat)..m3
- u f = Specific Internal Energy of saturated liquid ....(units kJ/kg)
- u fg = Specific Internal Energy of change of phase form liquid to gas at stated condions ..kJ/kg
- u g = Specific Internal Energy of dry saturated gas (No liquid content-but no superheat)..kJ/kg
- h f = Specific Internal Energy of saturated liquid ..kJ/kg
- h fg = Specific Latent heat or Enthalpy of change of phase..kJ/kg
- hg = Specific Enthalpy of dry saturated gas (No liquid content-but no superheat) ..kJ/kg
- s f = Specific Entropy of saturated liquid ..kJ/kg.K
- s fg = Specific Entropy of change of phase form liquid to gas at stated condions ..kJ/kg.K
- s g = Specific Entropy of dry saturated gas (No liquid content-but no superheat)..kJ/kg.K
|
When using steam tables it is often necessary to determine to property of a steam/water mixture. In this case
the quality (x) of the steam is used. This is defined as the mass of steam per unit mass of the mixture. The following relationships apply.
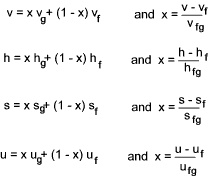
|